Acid Base Titration Experiment Report
Find the concentration of NaOH in molarity M. They may be weak acids that dissociate and change colour in alkaline solutions.

Pdf Che 226 Analytical Chemistry Laboratory 40 Acid Base Titration Experiment 7 Identifying A Substance By Acid Base Titration Safety Warning Raziol Oji Academia Edu
Neutralization between a strong acid HCl and a strong base NaOH is represented by H Cl- Na OH- Na Cl- H 2 O It is evident from the above equation that as NaOH solution is gradually added the H ion having high ionic conductance are replaced by Na having lower ionic conductance and hence the conductivity of the solution gradually decrease.

. Hydrochloric acid is a monoprotic acid. To prepare a standard solution of oxalic acid. To acquire the correct technique of titration.
In order to get access to kissing-prone sequences we tailored an experiment Figure Figure1A 1A using a synthetic library of RNA hairpins with an identical six base pair stem and a random loop sequence either 10 or 11 nucleotides long Figure Figure1B. The procedure was optimistically hailed by the authors as a useful synthetic method. 23 2013 42 likes.
It is a sensitive indicator for titration of weak organic bases and ammonia. Multiple samples release of volatiles can negatively impact. A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.
Titration experiment report rollaamalia. The electrophoresis mobility shift assay EMSA is a rapid and sensitive method to detect proteinnucleic acid interactions 123456It is based on the observation that the electrophoretic. Denoted is a quantitative measure of the strength of an acid in solutionIt is the equilibrium constant for a chemical reaction known as dissociation in the context of acidbase reactionsThe chemical species HA is an acid that dissociates into A the conjugate base of.
Back to Home Page. This pool displays a complexity of 52 10 6 candidates. An acid dissolves in water to show acidic properties which are H ions.
The acid-base indicator indicates the endpoint of the titration by changing colour. Some teachers report yellow with sodium hydroxide purple. Recommended Beer Degassing Methods and Alternatives Matrix.
The degassing of beer is a critical sample-preparation step for many beer analyses. On the report sheet record the initial reading of the NaOH solution in the buret to the nearest 002 ml. This experiment is to standardize the acid which is to identify its actual concentration.
Acid-Base Titration Determination Of The Concentration Of Hydrochloric Acid Solution. A base is a solution that reacts with acids to form salts. To determine the concentration of HCl solution.
Ask your instructor to check your reading and initial your report. Place a white sheet of paper under Erlenmeyer flask 1 to facilitate the detection of the end point when noting the color change of the indicator. The hot water or steam under pressure is then usable for transferring the heat to a process.
Alkali loads such as diets high in fruits and vegetables or potassium alkali supplements reduce uptake and increase urine citrate. Although multiple options exist for degassing each has its own advantages and disadvantages including degassing time cost throughput one sample vs. Acid base titration 1 Student.
Much later Ogura and co-workers modified the reaction conditions essentially by controlling the temperature between 10 C and 20 C of Sakurais experiment and obtained the same Grignard reagent in yields above 90 by titration 82CL1697. A molecule may be homonuclear that is it consists of atoms of one chemical element as with two atoms in the oxygen molecule O 2. Titration of 0824 g of potassium hydrogen phthalate required 38314 mL of NaOH to reach the endpoint detected by phenolphthalein.
LOG IN 0 ITEMS. Rough trial Trial 3 Trial 2 Trial 1 Initial reading mL Z10SML oO0mL O00ML o00ML Final reading mL 4315 mL 21oom L Z145ML Z150mL Volume dispensed mL 2155ML 21Lo5mL 210OmL 21SOML Average volume of NAOH used Trials 1-3. In the titration of ActD concentration experiment cultures of HEK cells in six-well plates transfected with either 1 μg of pCDNA33-EGFP only negative control or with 1 μg of pCDNA33-EGFP.
How to write a plan and design experiment Feb. This experiment uses the titration method. For some candidates.
Or it may be heteronuclear a chemical compound composed of more than one element as with water two hydrogen atoms and one oxygen. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. Get 247 customer support help when you place a homework help service order with us.
Acid loads such as high protein diets will increase citrate uptake into the renal cells and thereby reduce urine citrate. In addition to the sample an appropriate indicator is added to the titration chamber reflecting the pH range of the equivalence point. Perform an Acid Base Titration conniebisesi 1 of 2.
In chemistry an acid dissociation constant also known as acidity constant or acid-ionization constant. 2 A solution of NaOH was standardized by titration of a known quantity of the primary standard potassium hydrogen phthalate KHP FM 204221 gmol. Experiment 565 Prepare methyl red acid-base indicator 1.
Acid-base indicators change colour in acidic or basic solutions. Urine Citrate Varies With Acid Base Status. Sodium Carbonate in the other hand is a base.
To standardise 02 M NaOH solution. Acid-base titrations depend on the neutralization between an acid and a base when mixed in solution. A boiler is an enclosed vessel that provides a means for combustion heat to be transferred into water until it becomes heated water or steam.
ACID-BASE TITRATION Sample Data Collection and Results Pages Volume of NaOH used in the Titration.
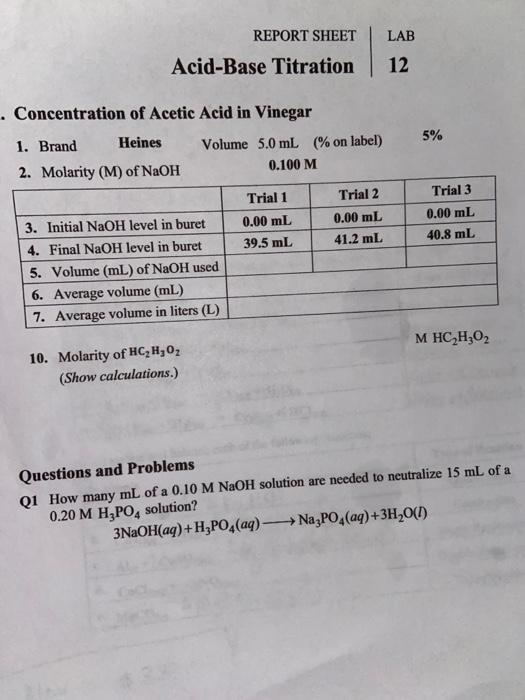
Solved Lab Report Sheet Acid Base Titration 12 Chegg Com

Acid Base Lab Report International Baccalaureate Chemistry Marked By Teachers Com

Experiment 17 Lab Report On Acid Base Reactions And Titration Lab Reports Chemistry Docsity

Acid Base Titration Lab Report The Purpose Of This Experiment Is To Determine The Concentration Of A Solution Of Sodium Hydroxide By Titration Against A Standard Solution Of Potassium Hydrogenphtalate International


0 Response to "Acid Base Titration Experiment Report"
Post a Comment